Insoles versus Orthotics versus Bio-Engineered Devices
The Confusion
- There Orthotic and Insole Markets are very confusing to both the patients as well as the doctors. Over the last 10 years, there has bee a steady and progressive influx of products which are called "Orthotics" but are merely standard off the shelf devices to manipulated or altered devices.
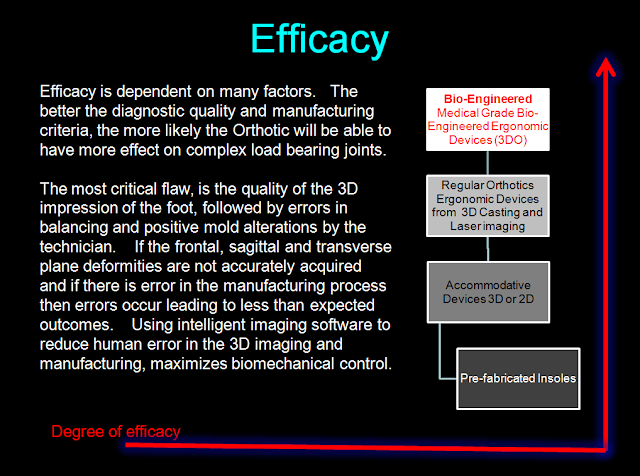
Efficacy is dependent on Proper Diagnosis and translating that diagnosis into a proper corrective solution(s)
The Junk Market
- The word "Orthotic" is a functional device that alters mechanical function. Strictly applied, it is a device or set of devices which normalizes function. Good functional control is dependent on the quality of biomechanical control needed to resolve a pathomechanical correction. Unfortunately, many products are called "Orthotics" and are either custom or altered. Case in point, are Dr Scholls Orthotics sold in pharmacies. Their 2D sensor system simply looks at static data (foot size) and drives an off the shelf product that is not corrective. Although they use "colors" to show 2D pressure, this data is not used to determine the off the shelf orthotic. The same can be said of Foot Levelers products which are really more Accommodative Orthotics due tot he fact that there is some information used from either a foam box or cast and now a 2D optical sensor system. They call it 3D, but frankly my investigation demonstrates that the optical imager is nothing more than a web camera and software that uses optical algorithms to assimilate a 3D image. The image is produced from estimations of Z Axis data and I do not find any geometry involved. I asked them to supply information, but they said it was a proprietary secret. As with many of these products, (and there are many), efficacy and correction are not part of the solution.
The Art Form - 27 Error Points in Standard Custom Manufacturing
- Practioner - Proper diagnosis(s) - Custom devices require a proper diagnosis in order to understand how the device should be modified to maximize load bearing control. Too many times, the laboratory is unaware of biomechanicals goals.
- Practioner - Positioning of the patient properly - Placing the patient in the proper position is critical to reduce the effects of extrinsic muscle groups changing neutral joint positioning. The patient many times has Functional Equinus and if these deforming shortages are not delat with, pronated casts results leaving poor results.
- Practioner - Thickness of plaster - Plaster thiskness can lead to enormous error, especially IF it makes it to the lab intact and is not crushed. When the lab pours the plaster to make the positive mold, the negative cast (weakened), then collapses and the result is a pronated cast and device.
- Practioner - Water Temperature - If cold, changes (slows) plaster hardening properties.
- Practioner - Proper Neutral Joint Positioning - Books can be written on this subject. Everyone has a different theory, or they simply do not care. This causes many errors in the final product. The Sub Talar Joint, Midtarsal Joints and 1st Ray need to be in a proper frontal plane alignment. Computations from X Rays and Range of Motion calculations as well as Static Stance measurements should be done. All of this creates errors. 3DO (3D Orthodynamics) uses a reproducible starting point (Static Stance) and we build to Neutral Position through the analysis of Static and Dynamic data relativre to 7 areas of data; (1) Mass Displacement analysis (2) Motion Analysis (3) Pressure Analysis (Linear and Sheer) (4) Body Balance in all 3 planes (5) Symmetry (6) Gait Analysis (7) 3D Geometry measurements (millimeters in XYZ axis)
- Patient movement - Many times unaware to the doctor, the patient moves their feet changing the alignment of the negative casts. The results are obvious. . . errors
- Practioner - Plaster Cast drying time - This should be obvious. . . A wet cast can become distorted because the plaster has not hardened.
- Practioner - Proper removal of negative casts - This is a real "Art". . Shaking off the cast without the patient moving feet is full of errors.
- Practioner or Office - Proper filling of the Rx form - Most labs will tell you this is a major problem. Getting the doctor or staff to fill out the Rx form is a consistent problem. If the staff does it, they may not know the proper solution for the patients problem. With 3DO, we use either "Default Ordering" where the technicians at Digital Orthotics evaluate the Static and Dynamic problems and make the proper corrections based on function and selected footwear. We also have a "Point and Click" Accommodation and Modification section which is very detailed and easy to use.
- Practioner or Office - Proper packaging and protection for shipping - Cast unprotected can get crushed or bent. The end result is error. . .
- Laboratory - Getting Patient Names mixed up - Logistics
- Laboratory - Proper Vertical Balancing - Negative casts are vertically balanced prior to the positive pour of plaster. Yikes, lots of errors here. If the cast is bad, more errors.
- Laboratory - Too much water content in plaster - If the plaster is too wet, the negative cast will break down.
- Laboratory - Too much medial arch fill - If technicians put too much plaster in arch, it pronates the alignment and leads ro errors. . .
- Laboratory - Too much heel plaster build up - Same as 14
- Laboratory - Proper heating of thermoplastic - Thermoplastics can get over heated and under heated causing a variety or problems. If under heated, improper coaptation of plastic against shell positive can occur. If overheated, weakness and fatigue.
- Laboratory - Proper Suction Pressure to bladders - Proper suction negative pressure will cause problems with coaptation and or breakage of positive casts.
- Laboratory - Plastic Durometer Thickness
- Laboratory - Rough Grind Errors
- Laboratory - Final Grind Errors
- Laboratory - Proper Rearfoot Posting - A major set of problems here. . . Varus positioning is critical for good rearfoot to midfoot control. Intrinsic posts do not work as well as extrinsic posts due to surface area.
- Laboratory - Translating proper Rx from Physician (Footwear - Diagnosis)
- Laboratory - Understanding Functional Disease
- Laboratory - Proper placement of Accommodations and modifications
- Laboratory - Selecting Proper Top Covers
- Laboratory - Glue applications and proper drying
- Laboratory - Product detail and trimming of Extensions (1/2 - 3/4 - Full)
Four Manufacturing Methods used by Digital Orthotics
- Plaster Cast to Positive Corrective Mold Thermoforming (The Standard)
- CNC Milling
- Computed 3D Geometry to Shell Thermoforming
- Custom On Demand
Precision 3D Manufacturing
Digital Orthotics Lean Custom Manufacturing using Digital Imager Live Cloud Software
- Many of the errors in Neutral Joint Positioning (Plaster Art Form), have been eliminated using 3DO Imagers and Digital Imager Live Software which analyzes Static and Dynamic cyclic load bearing conditions and computes neutral positioning using intelligent software. According to Dr Craig Lowe, "load bearing function of the body against the floor is key to our understanding of biomechanical diseases". There are just too many unknown errors associated with Plaster, foam boxes and Laser Imaging primarily due to the inherent "Art" associated with the modality. Conditions such as Mass Displacement, Motion Analysis, Pressure Analysis, Body Balance, Symmetry, Gait analysis and 3D Geometry are critical for understanding the pathomechanics of diseases. From the simple to the complex biomechanical load pattern, 3DO technology provides the unseeable insight needed to manufacture a Bio Engineered Corrective Device that actually provides anatomical and physiological correction.
Laboratory Network - US and International
- Digital Orthotics is a Open Loop Manufacturing System which can incorporate a variety of methods as described above. We believe in Strategic Partnerships where we enable private Orthotic Laboratories to license our intellectual property for profit. License laboratories are able to purchase hardware and software and manufacture custom products under a mutual non-disclosure non-circumvention agreement.
Ortho Rite - New York
Mile High Orthotics - Colorado
Excel Biomechanics - Canada
Millennia Holdings - Japan
Millennia Holdings - Los Angeles, California
Foot Solutions - Atlanta Georgia
Digital Orthotics - Riverside
Mark Speilbauer Orthotics
Millennia Holdings - South Korea (in development)
Companies we are working with or have worked with;
- Fila Footwear
- New Balance
- Reebok
- CCM Hockey
- Adidas
- Spira Footwear
- Custom Sports Footwear
- Rocky Bootwear
- Foot Solutions
- Timberland
- Pony Footwear
- Canadian Digital Orthotics
- Rocky Mountain Orthotics
- Bio-Orthopedic Laboratory
- Foot Comfort Laboratory
- Fujitsu Electronics
- Southern California Edison
- Hawiian Dredging
- Werehauser Paper
- Tuttle Glick Automotive
- US Department of Defense - Pentagon
- VA Hospitals
- US Olympics
- NFL -National Football League Alumni
- Professional Athletes (see web site under testimonials)